Articles
Published
21 years agoon
Sure, you know how to coat mesh with a liquid photoemulsion. But do you know the chemistry and physics behind the resulting stencil thickness? Did you know that some emulsions are more readily adaptable to different mesh counts? And, did you know that solids content and viscosity aren’t the only factors that influence the stencil thickness you can achieve?
Most printers seem to have a grasp on the terms solids and viscosity, or at least they understand that these are important characteristics of liquid photoemulsions. But ask even an experienced printer to define another key emulsion variable–rheology–and their facial expression quickly turns to one of confusion. Solids, viscosity, and rheology are the three physical properties of a photoemulsion that affect the final stencil thickness we can achieve.
Of course, stencil thickness isn’t just a result of emulsion properties. Creating a stencil to achieve specific printing characteristics requires the correct combination of many factors, which can be divided into three broad categories:
1. coating variables, including type of coating trough, coating speed, pressure, etc.
2. mesh variables, such as thread count, thread diameter, size of mesh openings, screen tension, etc.
3. photoemulsion variables
In this discussion, we’ll focus on the last category–photoemulsion variables–and look closely at solids content, viscosity, and rheology. We’ll review what they are, where they come from, and how they influence the buildup of the stencil during the coating process.
Understanding emulsions
First, let’s review what a photoemulsion is. The scientific term “emulsion” refers to a suspension of particles in a liquid. Some common household examples of emulsions include mayonnaise, skin moisturizer, sun lotion, school glue, and paint. In the screen-printing industry, we often say “emulsion” when referring to photoemulsions. The negative-forming emulsions used in screen printing contain light-sensitive molecules that are suspended in a carrier medium, hence the name photoemulsion.
Ever hear the following statement on your production floor? “This emulsion is thick, so it must have high solids.” As a photoemulsion chemist, I cringe when I hear customers and salespeople equate high viscosity with high solids. The truth is, there is no direct correlation between viscosity and solids content.
While it is possible for a high-viscosity photoemulsion to also have high solids, the viscosity level is not necessarily a result of solids content. To understand why this is, we must first understand basic photoemulsion chemistry.
Chemical considerations
Given that photoemulsions are water-based, a coating will eventually dry, leaving behind any non-volatile (non-evaporative) compounds. We refer to these residual chemicals as “solids,” and these are what remain on the screen after drying. If a photoemulsion contains 40% solids, the remaining 60% is composed of water, and possibly a small amount of volatile solvents.
Some manufacturers use general references to solids content, such as “medium” and “high,” in describing the emulsions. Keep in mind that these terms do not reflect any industry standard–they are subjective descriptions given by the manufacturers. Medium solids content to one manufacturer may be considered high for another manufacturer. However, with current photoemulsion technology, it would be acceptable to label a photoemulsion with 35% solids as “high solids;” however, this could better be described as “average.” A few manufacturers raise the bar by providing emulsions with 45% solids or more. Of course, no manufacturer is going to tout “low solids,” which I would consider to be less than 30%.Marty Medvetz
Viscosity is the measure of a liquid’s resistance to flow. It is the result of molecular content, size, and structure, as well as intermolecular forces and temperature. Viscosity also can be subjectively expressed as water-thin or thick as molasses. Additionally, however, it can be quantified with an instrument called a viscometer. For reference, water is approximately 0 centipoise (cps), while pancake syrup comes in with around 20,000 cps at room temperature.
The quantity and structure of the polymers formulated into a photoemulsion have the greatest influence on the final solids content, viscosity, and rheology. Basic photoemulsions are mainly composed of two types of polymers: polyvinyl acetate (PVAc) and polyvinyl alcohol (PVOH). Although these are both vinyl polymers, they have different chemical and physical properties.
Polymers are formed by the free-radical polymerization of individual segments called monomers. This process is achieved in a controlled reaction environment where many small monomers are initiated to bond together in a repeating pattern, forming the long chain molecules called polymers. However, the final polymer molecules can exhibit variations–different functional groups can be attached. Therefore, numerous combinations of monomers can be reacted to form almost infinite variations of polyvinyl acetates and polyvinyl alcohols.
PVAc polymers are very large, organic molecules. In liquid suspension, these particles are large enough to block light; however, they are still too small to see, even with a magnifier. A PVAc emulsion typically looks and pours like milk–it is opaque white with water-like viscosity. Although PVAc emulsions can be diluted with water, they are usually manufactured with a solids content of 50% to 65%. A typical PVAc emulsion will have 55% solids content and a low viscosity of 1000 cps.
Dried PVAc films are generally pretty hydrophobic (that means water resistant for all of you without pocket protectors), but they are not solvent resistant. Since PVAc usually makes up the largest portion of a photoemulsion, it contributes the most towards the solids content.
PVOH polymers are the Yin to PVAc’s Yang. Particle sizes are much smaller; therefore, solutions look translucent and are pale in color. PVOH polymers have a functional group on the polymer chain that has a natural attraction to water. In other words, they are hydrophilic or water loving. Because of this molecular attraction to water, PVOH solutions tend to form a gel-like liquid and become very thick, even at a very low solids content. It would not be impossible for a 20% solids PVOH emulsion to reach a very high viscosity of 50,000 cps or more, like honey left in the refrigerator.
In contrast to PVAc, PVOH is very solvent resistant, not water resistant. Basic plastisol-ink blockout products are really nothing more than PVOH solutions with color and performance modifiers. A comparison of the primary characteristics of both PVAc and PVOH emulsions appears in Figure 1.
| Figure 1 Characteristics of Polyvinyl Emulsions | ||
| PVAc | PVOH | |
| Clarity, color | Opaque, white | Translucent, pale |
| Typical viscosity | Water like | Honey like |
| Typical solids content | 50-65% | 15-30% |
| Chemical resistance | Water resistant | Solvent resistant |
| Stencil effect | Good film thickness | Easy stencil development |
Common diazo emulsions combine both PVAc and PVOH chemistries. The type of ink the stencil is intended to resist will dictate the ratio of PVAc to PVOH. Water-resistant diazo photoemulsions will have a higher PVAc-to-PVOH ratio than solvent-resistant diazo emulsions, which require additional PVOH. Therefore, higher solids content and lower viscosity is typically found in water-resistant diazo emulsions when compared to their solvent-resistant counterparts.
Exceptions to the rule do exist. Besides PVAc and PVOH selection, formulators have at their disposal a number of ways to manipulate viscosity and solids content. Viscosity modifiers are available that can turn the same 50% solids PVAc solution from a watery 100 cps to a full-bodied 40,000 cps with 51% solids. Solids content can also be manipulated with inert fillers–powders such as starch and wood flour. These types of fillers are a cheap way to raise solids; however, they can play havoc on exposure times and stencil resolution.
A photoemulsion manufacturer can put together almost any combination of solids and viscosity (low solids with low viscosity, low solids with high viscosity, high solids with low viscosity, or high solids with high viscosity). Therefore, don’t assume your thick photoemulsion has high solids! Ask your sales representative for a technical-data sheet that clearly states the solids content.
The third dimension: rheology
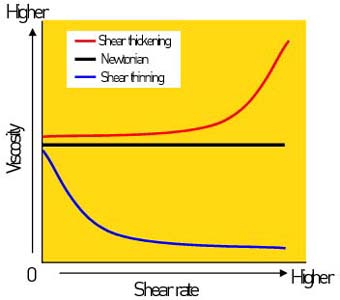
Can’t get that ketchup to come out of the bottle? Shake it! You’ll witness a phenomenon called shear-thinning rheology. Rheology is the science of flow and deformation of matter as it is subjected to changing stress. In simplest terms, it describes how the viscosity of a liquid will change as the liquid is moved. Although there are many classes of rheology that describe different scenarios, we will consider the three most basic types that apply to photoemulsions, all of which are depicted in Figure 2.
Shear thickening Viscosity increases (liquid thickens) as shear stress increases. As the liquid is forced to move faster, its resistance increases. Liquids with this property are described as rheopectic. Examples include corn starch in water, clay slurries, and wet sand.
Newtonian Viscosity does not change upon change in shear stress. As the liquid is forced to move faster, its re-sistance does not change. Water falls in this category.
Shear thinning Viscosity decreases (liquid thins) as shear stress increases. As the liquid is forced to move faster, its resistance decreases. Liquids that exhibit this property are called thixotropic. Examples include ketchup, paint, shampoo, plastisol ink, and photoemulsions.
Explaining rheology
As you stir water faster and faster, the viscosity remains the same. However, you’ll wait a long time before that ketchup pours out of the bottle. When you begin to shake the bottle, the fluid begins to flow more easily; the faster you can shake it, the quicker it pours. The difference between water and ketchup is not only the initial viscosity, but also how that viscosity changes as the liquid begins to move. This is defined by the liquid’s rheology.
Many screen-printing inks exhibit a shear-thinning characteristic. The ink is thick (high viscosity) as you plop it onto the screen. As the squeegee moves the ink and pushes it through the mesh, thus shearing it, the ink becomes thinner (viscosity drops). Once the squeegee has passed and the screen is raised, removing the shear stress, the ink begins to return to its higher viscosity level. This characteristic allows ink to quickly flow into and conform to the stencil’s shape, get “cut” at the top surface by the squeegee, and then remain in the defined shape until cured.
Like inks, photoemulsions can also exhibit this shear-thinning effect. The emulsion in your coating trough begins at its highest viscosity. As the coater passes over the mesh, it forces the emulsion under the coating edge and through the mesh. At that moment, the photoemulsion’s viscosity drops, allowing it to quickly flow through the mesh openings. When the coater is removed, the emulsion begins to revert back to its original, higher viscosity.
Different photoemulsions exhibit different shear-thinning profiles. Even with the same solids content and initial viscosity, two photoemulsions may not create the same stencil thickness. If we were to coat these two emulsions onto a high mesh count, say 355 threads/in., the emulsion with the higher shear-thinning rate would build a thicker stencil. This higher shear-thinning rate would allow the emulsion to more readily flow through the mesh and lead to a greater amount of emulsion on the screen.
On low mesh counts, differences in rheology profiles are much more noticeable. Figure 3 shows the result of coating a screen with two SBQ photoemulsions that have similar solids content (blue = 52%, pink = 48%) and similar viscosities when stirred. A sharp-edge coater was filled at the same time with both emulsions, half with the blue emulsion, half with the pink emulsion. A 61-thread/in. mesh was coated two times on the print side, then five times on the squeegee side, all wet-on-wet. The screen was then allowed to dry with the substrate side down at 75°F (24°C) and 25% hu-midity with a fan circulating the air.
As is clear from Figure 3, the blue emulsion was able to produce a thick coating without dripping off the mesh. Its thickness becomes even more evident in Figure 4, which shows a magnified view of a stencil opening after the emulsion was dried, exposed, and developed. The reason for the stencil-thickness differences between the two emulsions can be explained by the differences in their shear-thinning profiles.
The viscosities of both emulsions drop to a similar level as they are coated onto the mesh, and then begin to return to their original viscosity after the scoop coater is removed. However, the differences between these two emulsions are the at-rest viscosity and the shear-thinning rate. As Figure 5 shows, the blue emulsion has a higher starting viscosity, but drops dramatically during coating, nearly matching that of the pink emulsion. After coating, shear stress is removed, allowing the emulsions to return to their original viscosity. Since the pink emulsion has a lower shear-thinning rate and lower at-rest viscosity, the intermolecular forces are unable to retain its mass before drying, resulting in an uneven dry coating.
Similarly, it is possible for a lower-solids photoemulsion to match or surpass stencil thickness of a higher-solids emulsion with the same viscosity. With greater shear-thinning ability, the lower-solids emulsion can allow for faster stencil build.
Note, however, that there is a limit to the speed at which each photoemulsion can be applied. Even with a highly shear-thinning product, it’s not recommended to coat 3 ft of 61 thread/in. mesh in less than one second–standard photoemulsions are not designed for high-velocity application. Coating mesh, especially 80 thread/in. or lower, at high speeds, has the potential to generate air pockets in the mesh openings, which usually leads to pinholes.
A photoemulsion’s rheology profile is the result of formulation combinations and subtleties within each raw material selected. As mentioned earlier, there are variations of PVAc and PVOH to choose from. It is highly unlikely that two different polymer manufacturers can produce identical polymers from the exact same monomers.
Focus on physical characteristics
Disregarding the human element, the final stencil thickness on a given mesh count will be most influenced by the photoemulsion’s physical characteristics, including solids content, viscosity, and rheology. Change any one of these variables, and stencil thickness will change. These characteristics are unique for each emulsion product and can especially differ from manufacturer to manufacturer.
As a quality printer, your goal should be to find the right product that offers the optimum combination of physical and performance features to consistently give you the perfect stencil for your application. Simply matching solids and viscosities of two different photoemulsions is no guarantee that you’ll get identical stencils.
Marty Medvetz is technical manager for the Screen Graphics Division at Franklin Int’l, a Columbus, OH-based manufacturer of photoemulsions, adhesives, glues, sealants, and other materials. A chemist, Medvetz formulates photoemulsions for stencilmaking, screen-printable adhesives, and related products. He can be reached through Franklin International’s Website at www.imagemate.com.

Subscribe

Magazine
Get the most important news
and business ideas from Screenprinting Magazine.
Most Popular
-

 Art, Ad, or Alchemy1 month ago
Art, Ad, or Alchemy1 month agoF&I Printing Is Everywhere!
-

 Case Studies1 month ago
Case Studies1 month agoHigh-Density Inks Help Specialty Printing Take Center Stage
-

 Andy MacDougall1 month ago
Andy MacDougall1 month agoFunctional and Industrial Printing is EVERYWHERE!
-

 Columns2 weeks ago
Columns2 weeks ago8 Marketing Mistakes Not to Make When Promoting Your Screen Printing Services Online
-

 Editor's Note2 weeks ago
Editor's Note2 weeks agoLivin’ the High Life
-

 Thomas Trimingham2 months ago
Thomas Trimingham2 months ago“Magic” Marketing for Screen Printing Shops
-

 Marshall Atkinson2 weeks ago
Marshall Atkinson2 weeks agoHow to Create a Winning Culture in Your Screen-Printing Business
-

 News & Trends1 month ago
News & Trends1 month agoWhat Are ZALPHAS and How Can You Serve Them in Your Print Business?






